Video Article Open Access
Human Tissue Models for Advanced Bioevaluation of Antimicrobial Biomaterials-Based Technologies
Jochen Salber1,2,*, Ayesha Idrees1,2,3, Dierk Gruhn1,2, Gianluca Ciardelli3, Richard Viebahn1, Valeria Chiono3
1Universitätsklinikum Knappschaftskrankenhaus Bochum, Department of Surgery, Hospital of the RUHR-University, 44892 Bochum, Germany
2Department of Experimental Surgery, Centre for Clinical Research, RUHR-University, 44801 Bochum, Germany
3Department of Mechanical and Aerospace Engineering (DIMEAS), Politecnico di Torino, 10129 Turin, Italy
Vid. Proc. Adv. Mater., Volume 3, Article ID 2208331 (2022)
DOI: 10.5185/vpoam.2022.08331
Publication Date (Web): 13 Dec 2022
Copyright © IAAM
Graphical Abstract
Abstract
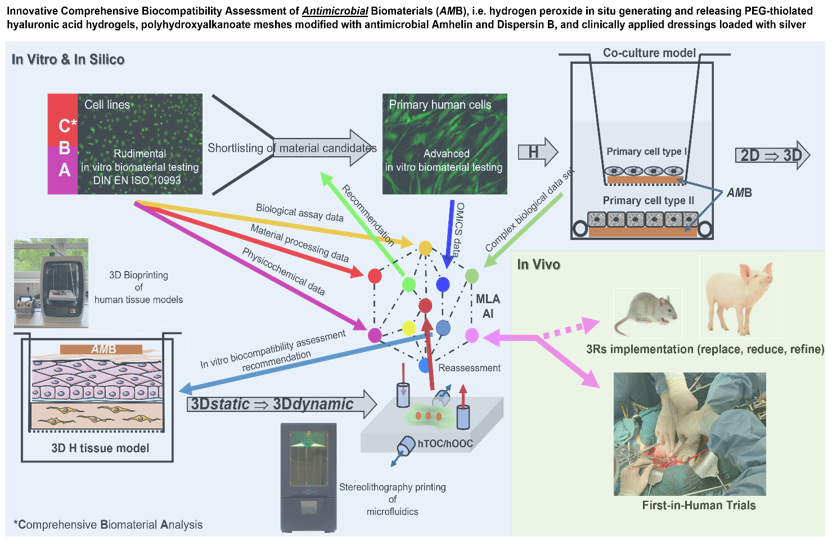
Introduction: Highly resistant bacteria and fungi in particular have led to an international resistance crisis in recent decades, which was relatively underestimated for a long time. At the same time, the development of new antibiotics and antimycotics was criminally cut back. This seriously jeopardises the maintenance of modern high-performance medicine and in particular the use of invasive biomaterial-based medical devices. This is where the many new developments in materials science can help to develop so-called complementary antimicrobial strategies to protect implants from microbial colonisation and biofilm formation. Before these antimicrobial biomaterials can be used, for example, as release systems, implant coatings or as temporary scaffolds in humans, their biocompatibility must be proven preclinically. With the help of today's biofabrication and information processing technologies, biocompatibility testing of antimicrobial biomaterials (AMBs) is becoming more comprehensive, faster and cheaper. The innovative and comprehensive assessment of the biocompatibility of AMBs is illustrated by H2O2 releasing, biodegradable hydrogels [1], Amhelin/Dispersin B (DB) modified polyhydroxyalkanoate (PHA) meshes [2], and Ag+ releasing dressings [3]. A sufficient killing effect against highly resistant bacteria is one thing, but the simultaneous guarantee of high cell, blood and immune system compatibility in humans, preclinically tested on human 3D tissue equivalents [4,5], must be ensured before the first clinical trials in humans can be conducted.
Materials and methods: Hyperbranched PEG-thiolated hyaluronic acid hydrogels with the ability to generate/release H2O2 in situ were prepared by a thiol-ene click reaction between hyperbranched polyethylene glycol diacrylate (HB PEGDA) synthesised by reversible addition-fragmentation chain transfer (RAFT) polymerisation and thiolated hyaluronic acid (HA-SH). Honey mimetic properties were realised by hydrogel loading with glucose oxidase (GO). GO causes conversion of D-glucose to D-gluconolactone with release of H2O2. PHAs were produced by bacterial fermentation under nitrogen-limiting conditions and with glucose as carbon source using Pseudomonas mendocina CH50. PHA-[NCO-sP(EO-stat-PO)] based blend fibres functionalised with bacterial membrane targeting Amhelin and biofilm disrupting enzyme DB were prepared by electrospinning. Various silver-containing wound dressings were obtained from different suppliers. All biomaterials were extensively characterised chemically and biologically [1,2,3]. The guidelines of the International Organisation for Standardisation (DIN EN ISO 10993) and the European Committee for Antimicrobial Susceptibility Testing (EUCAST) were followed. The detailed protocols for a human 3D skin equivalent, its complete characterisation and the application of 3D cell/tissue culture-based assays can be found at Idrees et. al. [4,5].
Key findings: Using various AMB systems, it could be shown that well-established, fast-to-perform and less complex 2D cell culture assays for DIN-compliant in vitro biocompatibility analyses are not yet obsolete. The data can be used in the CBA phase for pre-selection of specific biomaterial candidates. As a result of the amount of material/modification-specific data and unpredictable correlations with the collected biological cell-biomaterial effects, machine learning algorithms can help with candidate selection. Nevertheless, it was confirmed that different AMB approaches under more physiologically realistic 3D tissue culture conditions resulted in less toxic reactions at higher antimicrobial compound concentrations. By using dynamic 3D culture systems, so-called on-chip tools, the cultivation period of the presented human 3D HSE could be significantly extended and thus longer-term pathological, physiological or regenerative effects of AMBs could be investigated and analysed.
Major conclusions: Dynamic 3D tissue culture offers much more realistic investigation possibilities. Considering the higher costs for biocompatibility analysis with hTOC systems, MLA and AI concepts can be helpful in the selection of AMB candidates.
Keywords
Antimicrobial resistance; biomaterials assessment; human tissue models; organ-on-a-chip; 3D printing.
Acknowledgement
This work was financially supported by the European Union’s Horizon 2020 Research and Innovation Program, Marie Sklodowska-Curie Grant Agreement No. 643050 (HyMedPoly Project).
References
- J. M. Vasquez, A. Idrees, I. Carmagnola, et al., Frontiers in Bioengineering and Biotechnology, 2021, 9, 742135.
- S. Piarali, L. Marlinghaus, R. Viebahn, H. Lewis, M. G. Ryadnov, J. Groll, J. Salber, I. Roy, Frontiers in Bioengineering and Biotechnolgy, 2020, 8, 442.
- P. Varela, L. Marlinghaus, S. Sartori, R. Viebahn, J. Salber, G. Ciardelli, Frontiers in Bioengineering and Biotechnology, 2020, 8, 124.
- A. Idrees, I. Schmitz, A. Zoso, D. Gruhn S. Pacharra et. al., 4open, 2021, 4, 1.
- A. Idrees, V. Chiono, G. Ciardelli, S. Shah, R. Viebahn, X. Zhang, J. Salber; International Journal of Artificial Organs, 2018, 41, 779.
Biography
J. Salber holds a PhD in polymer chemistry and biomedical sciences of the RWTH Aachen University, Germany and is a board-certified orthopedic surgeon with 14 years of clinical experience working as emergency doctor and trauma surgeon. He is a full instructor of the European Resuscitation Council (ERC) and educates future ER specialists. Currently, he is head of the Department of Experimental Surgery at the Center for Clinical Research at the Ruhr University Bochum (RUB) and a senior physician at the Department of Surgery at the University Hospital RUB, Germany. His clinical interests are focused on Foreign Material-Associated Infections (FMAI), sepsis and multiple trauma immunology. His research group is working in the fields of clinically applied biomedical research with focus on biocompatibility assessment, tissue engineering and regenerative medicine. Since 2021, Dr Salber has been teaching at the RUB in the research focus Molecular Medicine on the topic “Molecular regulation of biomaterial-cell/tissue interactions: How can biocompatibility be accurately tested to avoid implant failure?“.Refereed Journal Publications >60; h-index: 22; i10-index: 29; Citations: 1776; RI-score: 1114.
Video Proceedings of Advanced Materials

Upcoming Congress



