Video Article Open Access
Biofunctionalization of Metals
Takao Hanawa1,2
1Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, Tokyo, 101-0062, Japan
2Center for Advanced Medical Engineering Research and Development, Kobe University, Kobe, 657-8501, Japan
Vid. Proc. Adv. Mater., Volume 3, Article ID 2206346 (2022)
DOI: 10.5185/vpoam.2022.06346
Publication Date (Web): 23 Dec 2022
Copyright © IAAM
Graphical Abstract
Abstract
Approximately 80% of implant devices are made of metal, and metals therefore continue to play an important role in medical treatment. Current research efforts in metallic biomaterials are made with the following categories: the elucidation of interfacial reactions between metals and tissues (including evaluations of safety and corrosion resistance); the development of new alloys; the development of new surface treatment techniques (including the control of surface morphology) [1]. The elucidation of the biocompatibility and biofunction of materials are important to develop excellent biomaterials. To add biofunctions to metals, new alloy designs and many techniques for the surface modification have been investigated. In particular, surface treatment is an important tool to add biocompatibility and biofunction to metals.
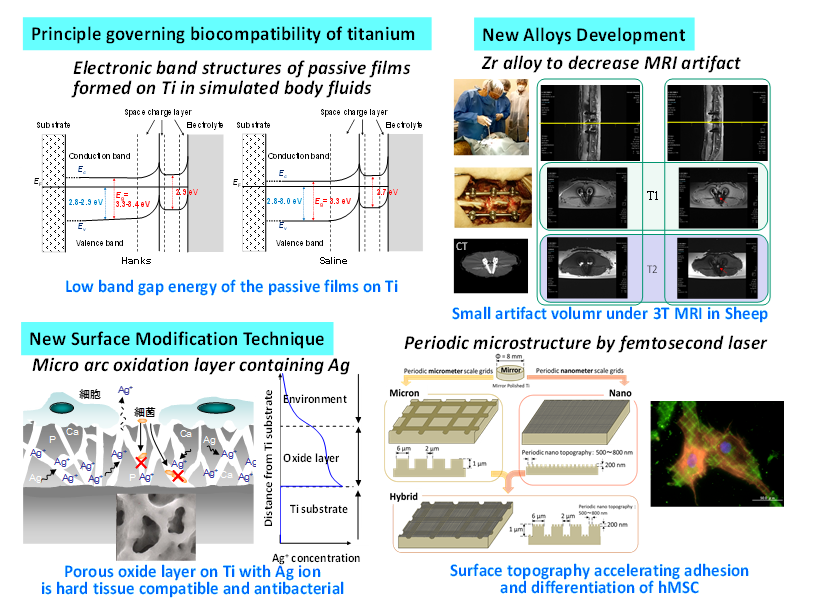
Ti and its alloys are widely used as implant devices in medicine and dentistry because of their excellent corrosion resistance and high specific strength. However, the principle and mechanism of the good tissue compatibility of Ti among metals have not been completely elucidated, despite numerous studies conducted on biological reactions. The band structures and band gap energies, Eg, of passive films formed on Ti in simulated body fluids were evaluated. The surface passive film on Ti showed a small Eg, so the Ti surface is more reactive with the surrounding environment than the TiO2 ceramic surface. This is probably one of the reasons for the excellent biocompatibility of Ti among metals, in addition to its excellent corrosion resistance [2].
Metals implanted into the human body have sometimes higher magnetic susceptibility than the surrounding tissues. Therefore, magnetic resonance imaging (MRI) diagnosing is disturbed by artifacts appearing on the MR images. In our previous study, Zr-1Mo alloy with low magnetic susceptibility is developed and generates much smaller artifact than Ti-6Al-4V ELI alloy. In addition, Zr-14Nb-5Ta-1Mo alloy has excellent balance of mechanical property with low magnetic susceptibility, high corrosion resistance, and no cytotoxicity. This alloy achieves large strength and elongation with small Youngs’ modulus and magnetic susceptibility that is not acquired in conventional Ti alloys [3].
After micro-arc oxidation (MAO), bone formation on titanium is accelerated. Antibacterial elements, silver, copper, and zinc are easily contained in the surface oxide layer by the addition of the elements in the electrolyte for MAO. To achieve a dual-functional surface both with bone formation and antibacterial properties, a sufficient quantity of Ag, Cu, and Zn are added into the electrolyte for MAO. An effective concentration of Ag, Cu, and Zn for the dual function is appeared. In this concentration region, both the osteogenesis and antibacterial activity appears [4].
To clarify the effects of micron/nano-meter level topography on cell behavior and morphology, we investigated the adhesion of human mesenchymal stem cell (hMSC) to Ti surfaces with micron/nano grooves and grids which created using a femtosecond laser. The different surface features had different effects on the differentiation of hMSC [5]. Metal surface is possibly biofunctionalized by various surface modification techniques. These techniques make it possible to apply metals to a scaffold in tissue engineering and regenerative medicine.
Keywords
Metallic biomaterial; surface treatment; alloy design; biofunction; biocompatibility.
References
- T. Hanawa, Frontier in Bioengineering and Biotechnology, 2021, 7, 170.
- S.C. Kim et al., Science and Technology of Advanced Materials, 2022, 23, 322.
- M. Ashida et al., Materials Transactions, 2020, 61, 776.
- M. Shimabukuro et al., ACS Biomaterials Science and Engineering, 2019, 5, 5623.
- P. Chen et al., Journal of Biomedical Materials Research Part A, 2018, 106, 2736.
Biography
I am a Professor and Chair of the Department of Metallic Biomaterials, Institute of Biomaterials and Bioengineering (IBB), Tokyo Medical and Dental University from 2004. I graduated Department of Metallurgical Engineering, Hokkaido University and received his Ph.D. from Hokkaido University at 1989 and Tohoku University at 1998. I have experienced as Assistant Professor in Hokkaido University, Associate Professor in Tokushima University, and Deputy-in-General of Biomaterials Research Center, National Institute for Materials Science, Tsukuba. I am working as a Professor, Center for Advanced Medical Engineering Research and Development Kobe University, Japan, in addition to Professor, Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, Japan. Since October 2020, I have joined Council Member of Science Council of Japan. I have been working in the field of metal-based biomaterials for medical and dental devices. Investigation of interfacial phenomena between solid state surface and biological environment is life-work of me. Base on this phenomena, many surface modification techniques have been developed and the performance of the resultant materials are evaluated by cell culture and animal tests. On the other hand, I have developed new alloys and manufacturing processes: Nickel-free austenitic stainless steels, zirconium alloys with low magnetic susceptibility to decrease MRI artifact, and high pressure torsion process of titanium alloys for dental narrow implants.
Video Proceedings of Advanced Materials

Upcoming Congress



