Video Article Open Access
A Molecular Description of Graphene: A Bridge from Benzene to Graphene Bridging its Chemistry and Physics
Aristides D. Zdetsis
Department of Physics, University of Patras, Patra, 26500 GR, Greece
Vid. Proc. Adv. Mater., Volume 2, Article ID 2021-02123 (2021)
DOI: 10.5185/vpoam.2021.02123
Publication Date (Web): 06 Apr 2021
Copyright © IAAM
Graphical Abstract
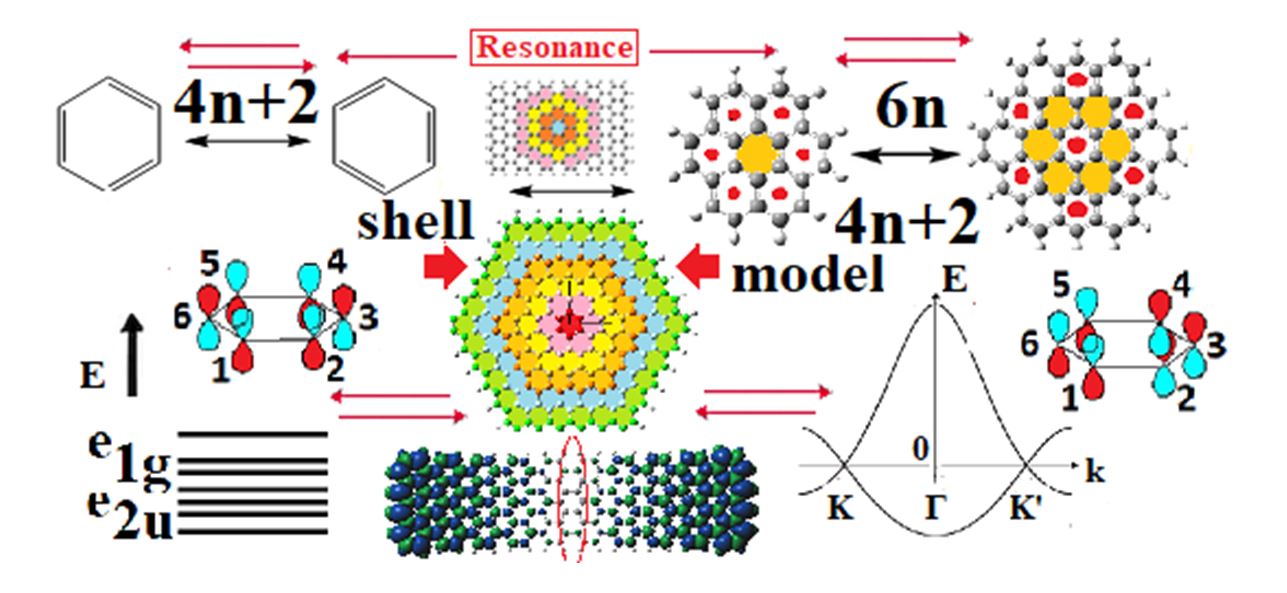
Abstract
The successful “molecular” description of graphene through a sequence of growing size polycyclic aromatic hydrocarbons (PAHs), not only serves as a bridge between the molecular and crystalline nature of graphene, but also at the same time links and interrelates in a transparent and comprehensive way the electronic and aromatic properties of benzene with those of graphene, revealing that graphene like benzene is a resonance structure, “resonating” between two complementary aromaticity patterns [1-2]. Thus, graphene is the prototypical aromatic “crystal” in full analogy to benzene which is the prototypical aromatic molecule. The two are connected through aromaticity, which is proven a very useful, simple, and transparent “everyday” tool, rather than a remote and “suspicious” concept [3]. The underlying fundamental linking property is the shell model (or shell structure), which can be visualized as a babushka-like Russian doll in which the PAHs of the main sequence [1] comprising the benzene-to-graphene bridge are stuck one inside the other. Through the shell model we can rationalize the two fundamental (but empirical) rules of aromaticity, Hückel’s and Clar’s, and establish their (topological) interconnection through the “shell model”. The shell structure, initially generated by hexagonal PAHs is fully operative in rectangular (D2h, and other symmetries) offering novel and vital understanding of the electronic properties of graphene nanoribbons (GNRs). Such understanding includes, among others, the 3n/ 3n±1 width rule for GNRs, as well as edge magnetism, which, if any, should not originate from real spin, but rather from pseudospin represented by the orientation of the atomic pz orbitals in the particular lattice site, which is fully determined by symmetry, and in particular by inversion symmetry. It is shown that inversion symmetry conflict in armchair GNRs (AGNRs) is responsible for the generation of zigzag edge states in AGNRs, which appear to be a subtle point in the experimental determination of the band gap in atomically precise AGNRs, in particular of the very narrow ones, together with other uncertainties [5]. On the other hand, we find that inversion symmetry conflict, which in the many-body theory of graphene leads to inversion (and parity) symmetry breaking, is responsible for the Dirac points of graphene, at the K points in the standard band structure which represents an “average picture” in relation to the “dynamical” picture of the present work, created by the spatial evolution of the hexagonal PAHs of the “main sequence” underlying the shell model [1]. Finally, in this work, trough the shell model and simple symmetry arguments, a new simple, transparent and powerful geometrical notion of aromaticity is revealed, which can be used as a “casual” modern tool, despite its past history and “suspicious behavior” [3], which coupled with simple symmetry arguments can lead to very rich, transparent, and insightful results, many of which could be obtained only by advanced techniques such as many-body and/or Chemical graph theory. The present approach should be also useful for other “honeycomb”-based structures, sharing the common feature of “alternant” or “bipartite” topology.
Keywords
Graphene; polycyclic aromatic hydrocarbons; aromaticity; nano graphene; graphene nanoribbons.
References
- D. Zdetsis, J. Phys. Chem. C, 2018, 122, 17526-17536
- D. Zdetsis, J. Phys. Chem. A 2020, 124, 976-986
- M. Sola, Front. Chem., 2017, 5, 22
- Zdetsis, J. Phys. Chem. C 2020, 124, 7578-7584
- Zdetsis, E.N. Economou, Carbon 2017, 116, 422-434
Biography
Aristides D. Zdetsis
Professor Emeritus Department of Physics
Division of Theoretical and Mathematical Physics, Astronomy and Astrophysics
Head of the Molecular Engineering Group
University of Patras, Greece
Education
1969-Bachelor’s Degree-Department of Physics University of Athens, GR
1972-Master’s Degree-Thomas Jefferson University, Swarthmore College Campus -Bartol Research Foundation, Swarthmore, Pa. USA. Dissertation: A review of the shell model with reference to the O18 nucleus.
1976-Ph.D-Degree -Thomas Jefferson University, Swarthmore College Campus -Bartol Research Foundation, Swarthmore, Pa. USA. Dissertation: Lattice Dynamics of Crystals with Diamond Structure.
Research Activities
Theoretical and Computational Condensed Matter Physics and Material Sciences, Nanoscience, Physical Chemistry and Quantum Chemistry. Nanophysics, Chemical Physics: Electronic, Optical and structural properties of amorphous semiconductors, clusters and nanostructures, graphene and graphene-based materials. There are about 155 publications 2000 citations, h factor 25, and 112 verified reviews) according to the web of science (see also google scholar referring to 2533 citations and h factor=29, https://scholar.google.gr/citations?user=oere_8UAAAAJ&hl=en
There are more than 60 presentations in Greek and international conferences (many of which are invited).
Books
- “Quantum Physics I” Ι» (pages 550) in Greek, Hellenic Open University (2004). ISBN: 960-538-626-7
- “Vibrations and Waves” (pages 377 Volume I) in Greek, Hellenic Open University (2004). ISBN: 960-538-629-1
- “Vibrations and Waves” (pages 338 Volume II) in Greek, Hellenic Open University (2004). ISBN: 960-538-630-5.
- “Introduction to Modern Physics” Lecture Notes (pages 600) in Greek, University of Patras (2003).
- “From Benzene to Graphene: Bridging The Physics and Chemistry of Graphene” 2020 (pending Publishing Agreement)
Video Proceedings of Advanced Materials

Upcoming Congress



